Modification of screen-printed electrodes with Prussian Blue (PB) and enzyme for diabetes control in sweat: Preliminary feasibility study
Modification of screen-printed electrodes with Prussian Blue (PB) and enzyme for diabetes control in sweat: Preliminary feasibility study
DOI:
https://doi.org/10.17488/RMIB.47.SI-TAIH.1521Keywords:
enzymatic sensor, glucose, non-invasive monitoring, prussian blue, screen-printed electrodeAbstract
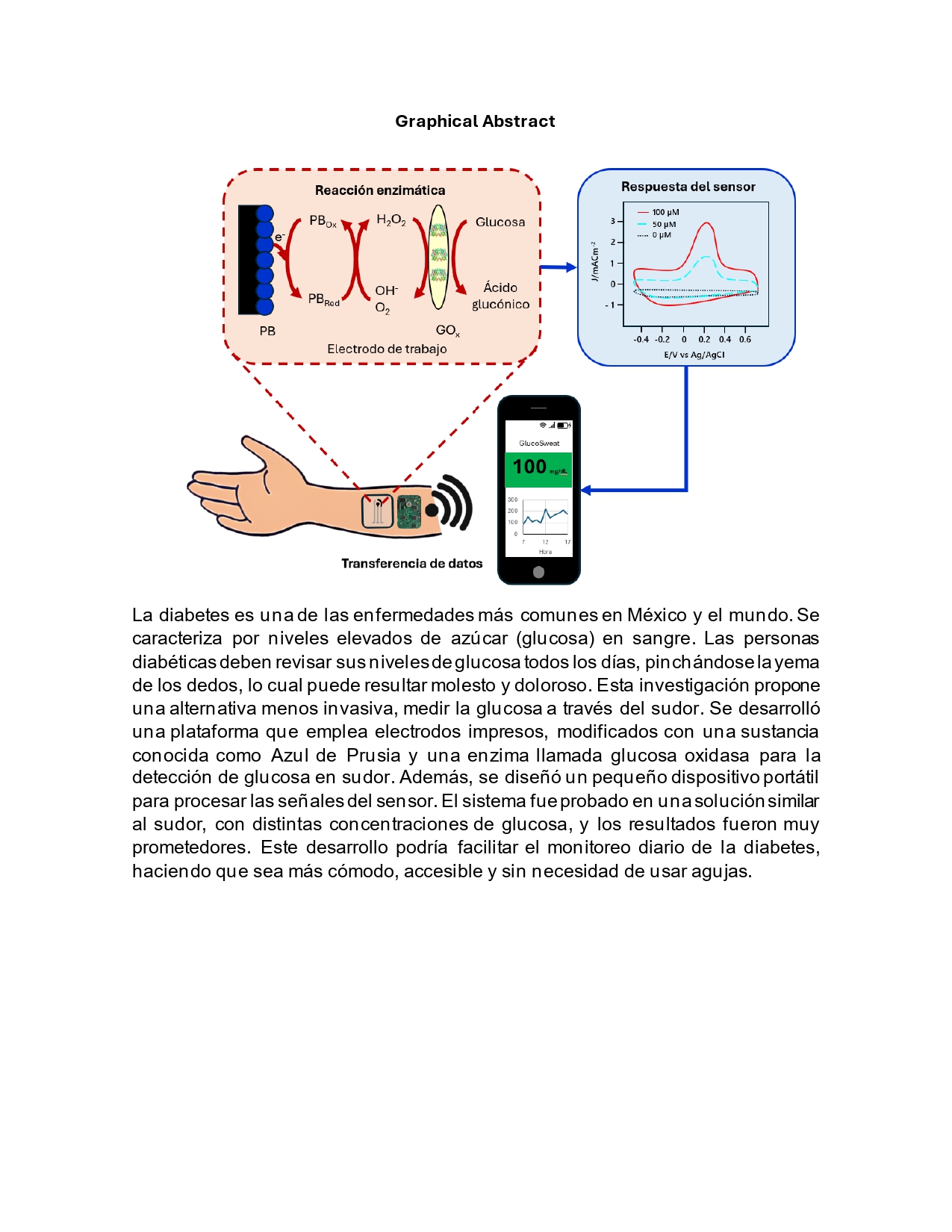
The prevalence of diabetes in Mexico has increased considerably over the past 20 years, thus increasing the need for the development of non-invasive and reliable glucose monitoring platforms. The present research focus on the development and evaluation of a novel non-invasive glucose monitor for the quantification of glucose levels in sweat. This platform is based on the use of screen-printed electrodes modified with a Prussian Blue (PB) electrochemical mediator and the glucose oxidase (GOx) enzyme to provide glucose specificity. Additionally, we present the design of a portable electronic device for generating the input signal and processing the analyte output signals. The system was evaluated in a 0.1 M phosphate buffered saline (PBS) solution supplemented with different physiologically relevant glucose concentrations. The results demonstrated that the PB mediator plays a key role in the detection of glucose, since the control electrode modified with the enzyme but lacking the PB mediator did not show trend, while the electrode modified with PB and enzyme showed a good correlation coefficient with analytical sensitivity of 0.0008 mA μM-1. This finding could revolutionize the field of glucose monitoring, facilitating more accessible and less invasive health technologies.
Downloads
References
A. Basto-Abreu et al., “Prevalencia de prediabetes y diabetes en México: Ensanut 2022,” Salud Publica Mex, vol. 65, pp. s163–s168, Jun. 2023, doi: https://doi.org/10.21149/14832.
Instituto Nacional de Estadística y Geografía, “Estadísticas de Defunciones Registradas (EDR), enero-junio de 2024.,” Ciudad de México, Jan. 2025.
M. Ortiz-Martínez, M. González-González, A. J. Martagón, V. Hlavinka, R. C. Willson, and M. Rito-Palomares, “Recent Developments in Biomarkers for Diagnosis and Screening of Type 2 Diabetes Mellitus,” Curr Diab Rep, vol. 22, no. 3, pp. 95–115, Mar. 2022, doi: https://doi.org/10.1007/s11892-022-01453-4.
National Institute of Diabetes and Digestive and Kidney Diseases, “¿Qué es la diabetes?” Accessed: Jan. 03, 2025. [Online]. Available: https://www.niddk.nih.gov/health-information/informacion-de-la-salud/diabetes/informacion-general.
J. B. Cole and J. C. Florez, “Genetics of diabetes mellitus and diabetes complications,” Nat Rev Nephrol, vol. 16, no. 7, pp. 377–390, Jul. 2020, doi: https://doi.org/10.1038/s41581-020-0278-5.
T. Kuzuya et al., “Report of the Committee on the classification and diagnostic criteria of diabetes mellitus,” Diabetes Res Clin Pract, vol. 55, no. 1, pp. 65–85, Jan. 2002, doi: https://doi.org/10.1016/s0168-8227(01)00365-5.
G. E. Umpierrez, M. B. Murphy, and A. E. Kitabchi, “Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar Syndrome,” Diabetes Spectrum, vol. 15, no. 1, pp. 28–36, Jan. 2002, doi: https://doi.org/10.2337/diaspect.15.1.28.
Secretaría de Salud, “547. En México, 12.4 millones de personas viven con diabetes,” Ciudad de méxico, 2022. Accessed: Feb. 06, 2025. [Online].
Available: https://www.gob.mx/salud/prensa/547-en-mexico-12-4-millones-de-personas-viven-con-diabetes?idiom=es.
J. Kim, A. S. Campbell, and J. Wang, “Wearable non-invasive epidermal glucose sensors: A review,” Talanta, vol. 177, pp. 163–170, Jan. 2018, doi:
https://doi.org/10.1016/j.talanta.2017.08.077.
J. P. Bantle and W. Thomas, “Glucose measurement in patients with diabetes mellitus with dermal interstitial fluid,” Journal of Laboratory and
Clinical Medicine, vol. 130, no. 4, pp. 436–441, Oct. 1997, doi: https://doi.org/10.1016/S0022-2143(97)90044-5.
A. Glasper, G. McEwing, and J. Richardson, Foundation Skills for Caring: Using Student-Centred Learning. Bloomsbury Publishing, 2018.
S. M. Khor, J. Choi, P. Won, and S. H. Ko, “Challenges and Strategies in Developing an Enzymatic Wearable Sweat Glucose Biosensor as a Practical Point-Of-Care Monitoring Tool for Type II Diabetes,” Nanomaterials, vol. 12, no. 2, p. 221, Jan. 2022, doi: https://doi.org/10.3390/nano12020221.
K. H. Cha, G. C. Jensen, A. S. Balijepalli, B. E. Cohan, and M. E. Meyerhoff, “Evaluation of Commercial Glucometer Test Strips for Potential
Measurement of Glucose in Tears,” Anal Chem, vol. 86, no. 3, pp. 1902–1908, Feb. 2014, doi: https://doi.org/10.1021/ac4040168.
J. R. Sempionatto, J.-M. Moon, and J. Wang, “Touch-Based Fingertip Blood-Free Reliable Glucose Monitoring: Personalized Data Processing for
Predicting Blood Glucose Concentrations,” ACS Sens, vol. 6, no. 5, pp. 1875–1883, May 2021, doi: https://doi.org/10.1021/acssensors.1c00139.
J. Moyer, D. Wilson, I. Finkelshtein, B. Wong, and R. Potts, “Correlation Between Sweat Glucose and Blood Glucose in Subjects with Diabetes,”
Diabetes Technol Ther, vol. 14, no. 5, pp. 398–402, May 2012, doi: https://doi.org/10.1089/dia.2011.0262.
M. Bariya, H. Y. Y. Nyein, and A. Javey, “Wearable sweat sensors,” Nat Electron, vol. 1, no. 3, pp. 160–171, Mar. 2018, doi: https://doi.org/10.1038/s41928-018-0043-y.
C. J. Harvey, R. F. LeBouf, and A. B. Stefaniak, “Formulation and stability of a novel artificial human sweat under conditions of storage and use,” Toxicology in Vitro, vol. 24, no. 6, pp. 1790–1796, Sep. 2010, doi: https://doi.org/10.1016/j.tiv.2010.06.016.
J. R. Sempionatto, J.-M. Moon, and J. Wang, “Touch-Based Fingertip Blood-Free Reliable Glucose Monitoring: Personalized Data Processing for
Predicting Blood Glucose Concentrations,” ACS Sens, vol. 6, no. 5, pp. 1875–1883, May 2021, doi: https://doi.org/10.1021/acssensors.1c00139.
S. Husmann, E. Nossol, and A. J. G. Zarbin, “Carbon nanotube/Prussian blue paste electrodes: Characterization and study of key parameters for application as sensors for determination of low concentration of hydrogen peroxide,” Sens Actuators B Chem, vol. 192, pp. 782–790, Mar. 2014, doi: https://doi.org/10.1016/j.snb.2013.10.074.
A. Hulanicki, S. Glab, and F. Ingman, “Chemical sensors: definitions and classification,” Pure and Applied Chemistry, vol. 63, no. 9, pp. 1247–1250, Jan. 1991, doi: https://doi.org/10.1351/pac199163091247.
K. A. Lamkin-Kennard and M. B. Popovic, “Sensors: Natural and Synthetic Sensors,” in Biomechatronics, Elsevier, 2019, pp. 81–107. doi: https://doi.org/10.1016/B978-0-12-812939-5.00004-5.
D. Citterio, “Chemical Sensor,” in Encyclopedia of Polymeric Nanomaterials, Berlin, Heidelberg: Springer Berlin Heidelberg, 2015, pp. 378–386. doi: https://doi.org/10.1007/978-3-642-29648-2_114.
L. Díaz de León-Martínez, J. Glöckler, B. Mizaikoff, R. Flores-Ramírez, and F. Díaz-Barriga, “Volatile Organic Compound Exhaled Breath Sensing,” in Encyclopedia of Sensors and Biosensors, Elsevier, 2023, pp. 421–440. doi: https://doi.org/10.1016/B978-0-12-822548-6.00154-0.
N. P. Shetti, D. S. Nayak, K. R. Reddy, and T. M. Aminabhvi, “Graphene–Clay-Based Hybrid Nanostructures for Electrochemical Sensors and Biosensors,” in Graphene-Based Electrochemical Sensors for Biomolecules, Elsevier, 2019, pp. 235–274. doi: https://doi.org/10.1016/B978-0-12-815394-9.00010-8.
I. L. de Mattos, L. Gorton, and T. Ruzgas, “Sensor and biosensor based on Prussian Blue modified gold and platinum screen printed electrodes,”
Biosens Bioelectron, vol. 18, no. 2–3, pp. 193–200, Mar. 2003, doi: https://doi.org/10.1016/S0956-5663(02)00185-9.
S. Kumar, S. Tripathy, A. Jyoti, and S. G. Singh, “Recent advances in biosensors for diagnosis and detection of sepsis: A comprehensive review,” Biosens Bioelectron, vol. 124–125, pp. 205–215, Jan. 2019, doi: https://doi.org/10.1016/j.bios.2018.10.034.
A. Abellán-Llobregat et al., “A stretchable and screen-printed electrochemical sensor for glucose determination in human perspiration,” Biosens Bioelectron, vol. 91, pp. 885–891, May 2017, doi: https://doi.org/10.1016/j.bios.2017.01.058.
R. Wilson and A. P. F. Turner, “Glucose oxidase: an ideal enzyme,” Biosens Bioelectron, vol. 7, no. 3, pp. 165–185, Jan. 1992, doi: https://doi.
org/10.1016/0956-5663(92)87013-F.
R. V. Blasques, J. S. Stefano, J. R. Camargo, L. R. Guterres e Silva, L. C. Brazaca, and B. C. Janegitz, “Disposable Prussian blue-anchored electrochemical sensor for enzymatic and non-enzymatic multi-analyte detection,” Sensors and Actuators Reports, vol. 4, p. 100118, Nov. 2022, doi: https://doi.org/10.1016/j.snr.2022.100118.
N. X. Viet, M. Chikae, Y. Ukita, and Y. Takamura, “Enzyme-Free Glucose Sensor Based on Micro-nano Dualporous Gold-Modified Screen-Printed Carbon Electrode,” Int J Electrochem Sci, vol. 13, no. 9, pp. 8633–8644, Sep. 2018, doi: https://doi.org/10.20964/2018.09.08.
K. Phasuksom and A. Sirivat, “Chronoampermetric detection of enzymatic glucose sensor based on doped polyindole/MWCNT composites modified onto screen-printed carbon electrode as portable sensing device for diabetes,” RSC Adv, vol. 12, no. 44, pp. 28505–28518, 2022, doi: https://doi.org/10.1039/D2RA04947C.
C.-L. Sun, W.-L. Cheng, T.-K. Hsu, C.-W. Chang, J.-L. Chang, and J.-M. Zen, “Ultrasensitive and highly stable nonenzymatic glucose sensor by a CuO/graphene-modified screen-printed carbon electrode integrated with flow-injection analysis,” Electrochem commun, vol. 30, pp. 91–94, May 2013, doi: https://doi.org/10.1016/j.elecom.2013.02.015.
F. Poletti et al., “Continuous capillary-flow sensing of glucose and lactate in sweat with an electrochemical sensor based on functionalized graphene oxide,” Sens Actuators B Chem, vol. 344, p. 130253, Oct. 2021, doi:. https://doi.org/10.1016/j.snb.2021.130253.
C. Espro, S. Marini, D. Giusi, C. Ampelli, and G. Neri, “Non-enzymatic screen printed sensor based on Cu2O nanocubes for glucose determination in bio-fermentation processes,” Journal of Electroanalytical Chemistry, vol. 873, p. 114354, Sep. 2020, doi: https://doi.org/10.1016/j.jelechem.2020.114354.
A. J. Bandodkar, W. Jia, C. Yardımcı, X. Wang, J. Ramirez, and J. Wang, “Tattoo-Based Noninvasive Glucose Monitoring: A Proof-of-Concept Study,” Anal Chem, vol. 87, no. 1, pp. 394–398, Jan. 2015, doi: https://doi.org/10.1021/ac504300n.
S.-H. Lee, H.-Y. Fang, and W.-C. Chen, “Amperometric glucose biosensor based on screen-printed carbon electrodes mediated with hexacyanoferrate–chitosan oligomers mixture,” Sens Actuators B Chem, vol. 117, no. 1, pp. 236–243, Sep. 2006, doi: https://doi.org/10.1016/j.snb.2005.11.028.
R. Harud, N. Most, veronica Preda, and N. Nasiri, “Advances in electrochemical sensors for real-time glucose monitoring,” Sensors & Diagnostics, vol. 3, pp. 893–913, 2024, doi: https://doi.org/10.1039/D4SD00086B.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2026 Revista Mexicana de Ingenieria Biomedica

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Upon acceptance of an article in the RMIB, corresponding authors will be asked to fulfill and sign the copyright and the journal publishing agreement, which will allow the RMIB authorization to publish this document in any media without limitations and without any cost. Authors may reuse parts of the paper in other documents and reproduce part or all of it for their personal use as long as a bibliographic reference is made to the RMIB. However written permission of the Publisher is required for resale or distribution outside the corresponding author institution and for all other derivative works, including compilations and translations.







